Answer:
A. increases
Step-by-step explanation:
According to the Gay-Lussac's law:-
Thus, at constant volume and number of moles, Pressure of the gas is directly proportional to the temperature of the gas.
P ∝ T
Also, it can be written as:-
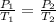
Thus, if the temperature is increased, the pressure exerted by the gas also increases.