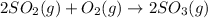Step-by-step explanation:
It is known that the oxidation of
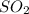 to
to
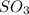 is catalyzed by
is catalyzed by
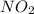 . In this process, two steps are involved as follows.
. In this process, two steps are involved as follows.
(a)
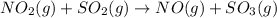
whereas, the second step is
(b)
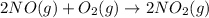
Balancing the above reaction by multiplying equation (a) by 2 as follows.
(c)
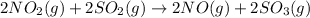
Now, on adding both reaction equations (b) and (c) the net reaction equation will be as follows.
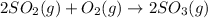
Thus, we can conclude that the equation for the final overall oxidation of
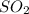 by
by
 to give
to give
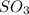 is as follows.
is as follows.