Step-by-step explanation:
According to Bronsted-Lowry an acid is defined as the specie which is able to donate hydrogen ions when dissolved in water.
For example,
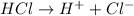
On the other hand, bases are the species which are able to donate hydroxide ions when dissolved in water.
For example,
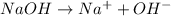
In
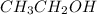 compound, when it will lose hydrogen ion then it will result in the formation of a
compound, when it will lose hydrogen ion then it will result in the formation of a
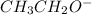 (alkoxide ion). As the carbon atoms are donating their positive charge towards the
(alkoxide ion). As the carbon atoms are donating their positive charge towards the
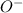 ion so, it will become unstable in nature.
ion so, it will become unstable in nature.
As a result, it will neither give a hydrogen ion or a hydroxide ion.
In
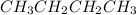 , there is no difference in the electronegativity of both carbon and hydrogen atoms. Therefore, this compound is not polar in nature hence, it will neither give a hydrogen or hydroxide ion.
, there is no difference in the electronegativity of both carbon and hydrogen atoms. Therefore, this compound is not polar in nature hence, it will neither give a hydrogen or hydroxide ion.
In
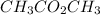 there is presence of no -OH group. Hence, it will neither give a hydroxide or hydrogen ion.
there is presence of no -OH group. Hence, it will neither give a hydroxide or hydrogen ion.
Thus, we can conclude that none of the given compounds will act as an acid or a base.