Answer:
0.084 M
Step-by-step explanation:
Using the Henderson-Hasselbalch equation for a buffer ( a buffer is solution contain a weak acid and it conjugate base; the solution resist change in pH)
pH = pKa + log ( base/acid)
4.9 - 4.76 =log ( base / acid)
10^0.14 = ( base / acid)
1.38 = (base / acid)
since there is 0.2 M in the buffer solution
the concentration of acid =
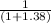 × 0.2 = 0.084 M
× 0.2 = 0.084 M