Step-by-step explanation:
Moles of phosphorus pentachloride present initially = 2.5 mol
Moles of phosphorus trichloride at equilibrium = 0.338 mol
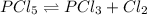
Initially
2.5 mol 0 0
At equilibrium:
(2.5 - x) mol x x
So, from above, the moles of phosphorus trichloride at equilibrium , x= 0.338 mol
Mass of 0.338 moles of phosphorus trichloride at equilibrium:
= 0.338 mol × 137.5 g/mol = 46.475 g
Moles of phosphorus pentachloride present at equilibrium :
= (2.5 - 0.338) mol = 2.162 mol
Mass of 2.162 moles of phosphorus pentachloride at equilibrium:
= 2.162 mol × 208.5 g/mol = 450.777 g
Moles of chloride gas present at equilibrium : 0.338 mol
Mass of 0.338 moles of chloride gas at equilibrium:
= 0.338 mol × 71 g/mol = 23.998 g