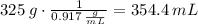Answer:
The volume of the water after it freezes (at 0°C) is 354.4 mL.
Step-by-step explanation:
We need to use this additional information, the densities of liquid water and ice at 0 °C are 1.000 g/mL and 0.917 g/mL, respectively.
Step 1. Find the mass of water before it freezes.
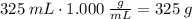
Step 2. The mass does not change when the temperature does. So, you take the grams of water and divide it by the density of the ice to obtain the volume it occupies.