Answer:
79.2 grams of oxygen gas are required to react with 22.50 grams of n-heptane.
Step-by-step explanation:
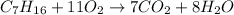
Mass of n-heptane = 22.50 g
Moles of n-heptane =
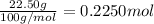
According to reaction 1 mole of n-heptane reacts with 11 moles of oxygen gas. then 0.2250 moles of n-heptane will react with:
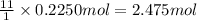 of oxygen gas
of oxygen gas
Mass of 2.475 moles of oxygen gas= 2.475 mol × 32 g/mol= 79.2 g
79.2 grams of oxygen gas are required to react with 22.50 grams of n-heptane.