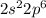Answer:
8 electrons
Step-by-step explanation:
Phosphorous is the element of group 15 and third period. The atomic number of chlorine is 15 and the symbol of the element is P.
The electronic configuration of the element phosphorus is:-
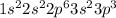
Thus, from the electronic configuration shown above,
In n = 2, the total number of electrons are 8 which are present in