Answer:
140 g (to 2 sf)
Step-by-step explanation:
The atomic mass of magnesium is 24.305 u.
The atomic mass of oxygen is 15.9994 u.
So, the gram-formula mass of magnesium dioxide, in g/mol, is
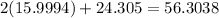
So, the mass of 2.5 moles is (56.3038)(2.5), which is equal to 140 g (to 2 sf)