The given molecule C_12 H_22 O_11 is sucrose.
Sucrose of not dissociable in water hence Vant Hoff factor is 1
i.e
- i=1
- molality=m=0.3
- k=freezing point constant (As we don't need to find the perfect freezing point let's keep it constant)

For Sucrose
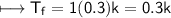
Now.
Let's check Vant Hoff factor of each compound.
- AlCl_3=4
- CuCl_2=3
- NaCl=2
- C_6H_12O=1
Let's count Freezing point of each compound.
AlCl_3
m=0.075
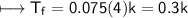
CuCl_2
m=0.15
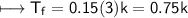
NaCl:-
m=0.3
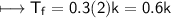
Glucose:-
m=0.6
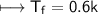
Hence option A is correct