Given that –
We know that –
Then –

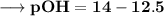

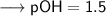

![\sf \longrightarrow [OH^- ] = 10^(-1.5 )](https://img.qammunity.org/2022/formulas/chemistry/college/5mhh5u0i2ohkqrfx1nd9ln5f1nfje1hh5x.png)

![\bf \longrightarrow [OH^-] = 3.16 × 10^(-2)](https://img.qammunity.org/2022/formulas/chemistry/college/cvuc5gknrxsyjuohurffvmahuzafsas00n.png)
Now the number of moles of KOH need to ensure that concentration of Hydroxide anions is equal to –

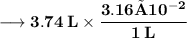

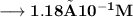
Volume of the solution contains the need number of moles of Hydroxide anions –

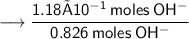

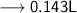

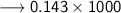

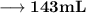
______________________________________