Final answer:
The balanced chemical equation for the formation of gaseous hydrogen bromide (HBr) from its elements in their standard states is ½
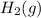 + ½
+ ½
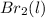 →
→
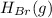 .
.
Step-by-step explanation:
To write a balanced chemical equation for the standard formation reaction of gaseous hydrogen bromide (HBr), you need to know the standard state of its elements at 25°C and 1 atmosphere. In this case, hydrogen (H2) is a gas, and bromine (Br2) is a liquid. The formation of HBr from these elements can be represented as follows:
½
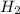 (g) + ½
(g) + ½
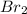 (l) →
(l) →
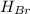 (g)
(g)
This equation shows that one mole of HBr gas is formed from one half mole of diatomic hydrogen gas and one half mole of diatomic bromine liquid, following the principle of the reaction of elements in their standard states to form the compound.