Answer:
0.1309 mol
Step-by-step explanation:
From the given information:
The metal ion, two ions of
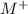 reacted with Cl⁻ to form
reacted with Cl⁻ to form
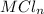 i.e. the compound formed is
i.e. the compound formed is
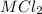 .
.
The concentration of the metal ion formed
![[M^+]](https://img.qammunity.org/2022/formulas/chemistry/college/tphonrkwh89sydg5nfxvkpjsbr74fx6csl.png) = 8.279 M
= 8.279 M
The concentration of the chlorine ion formed
![[Cl^-]](https://img.qammunity.org/2022/formulas/chemistry/college/7m8qslwty45fi84kteis6ix4i9c7h5uf87.png) = 2 × 8.279 M
= 2 × 8.279 M
= 16.558 M
∴
We know that:
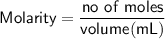
The number of moles of
![[Cl^-]](https://img.qammunity.org/2022/formulas/chemistry/college/7m8qslwty45fi84kteis6ix4i9c7h5uf87.png) =
=
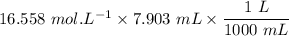
= 0.1309 mol