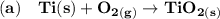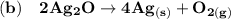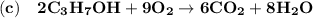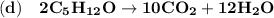Answer:
Step-by-step explanation:
A balanced chemical equation refers to the reaction taking place whereby the number of atoms associated in the reactants side is equivalent to the number of atoms on the products side.
From the given information, the balanced equations are as follows: