Solution :
We know that :
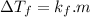 and
and
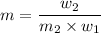
Then,
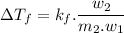 ..................(1)
..................(1)
Where,
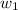 = amount of solvent (in kg)
= amount of solvent (in kg)
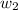 = amount of solute (in kg)
= amount of solute (in kg)
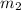 = molar mass of solute (g/mole)
= molar mass of solute (g/mole)
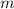 = molality of solution (mole/kg)
= molality of solution (mole/kg)
Given :
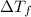 =
=
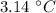 ,
,
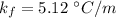
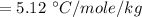
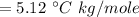
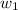 = 0.250 kg,
= 0.250 kg,
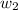 = 24.3 g
= 24.3 g
Then putting this values in the equation is (1),
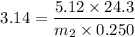
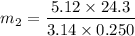
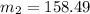 g/mole
g/mole
So, the molar mass of the unknown compound is 158.49 g/mole.