Answer:
25.88 g/mol
Step-by-step explanation:
Graham's law is a famous law which states that the diffusion rate or the effusion rate of any gas varies inversely to the square root of the molecular weight the gas.
So from Graham's law, we have,
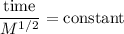
Using the sample of Kr gas having M = 83.8
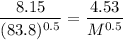
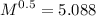
M = 25.88 g/mol