Answer:
The balanced chemical reaction is :
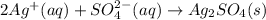
Step-by-step explanation:
The precipitation reaction is defined as a chemical reaction in which a solid substance is formed when two aqueous solutions of different compounds are allowed to react with each other.
When an aqueous solution of silver ion and sulfate ions are mixed together solid white precipitate of silver sulfate is formed.
The balanced chemical reaction is written as: