Answer:
About redox reaction which of the given statements are true?
Step-by-step explanation:
Redox reaction is the one in which both oxidation and reduction reactions take place simultaneously.
For example:
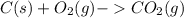
In this reaction, carbon undergoes oxidation and oxygen undergoes reduction simultaneously.
During this reaction, mutual exchange of electrosn take place between the oxidant and the reductant.
Among the given options,
Option B. electrons are transferred
and
option C.They include both oxidation and reduction takes place are the correct answers.