Answer:
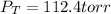
Step-by-step explanation:
Hello there!
In this case, since these problems about gas mixtures are based off Dalton's law in terms of mole fraction, partial pressure and total pressure, we can write the following for hydrogen, we are given its partial pressure:
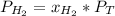
And can be solved for the total pressure as follows:
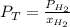
However, we first calculate the mole fraction of hydrogen by subtracting that of nitrogen to 1 due to:
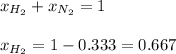
Then, we can plug in to obtain the total pressure:
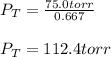
Regards!