Answer:
reaches a higher temperature is LEAD.
Step-by-step explanation:
In this exercise we have a calorimeter problem, in which the initial energy is absorbed by each body
E₀ = m c_e ΔT
where E₀ = 5000 J
ΔT =
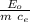
the specific heats for each material are tabulated
material c_e (J / kg ºC)
glass 837
copper 387
lead 128
gold 129
As they indicate that the mass of the materials is the same, the one with the greatest temperature difference is the one with the least specific heat, consequently the one that reaches a higher temperature is LEAD.
let's calculate for a mass of 1 kg
glass
ΔT = 5000/(1 837)
ΔT = 6ºC
Lead
ΔT = 5000/(1 128)
ΔT = 39.1º
GOLD
ΔT = 5000/(1 129)
ΔT = 38.8º C