Answer: For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equilibrium constant expression is K = [ H+] [NO2-] / [HNO2].
Step-by-step explanation:
An expression that depicts the ratio of products and reactants raised to the power of their coefficients at equilibrium is called equilibrium constant.
An equilibrium constant is denoted by the symbol 'K'.
For example, the dissociation of nitrous acid in aqueous solution is as follows.
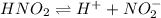
Hence, its expression for equilibrium constant is as follows.
![K = ([H^(+)][NO^(-)_(2)])/([HNO_(2)])](https://img.qammunity.org/2022/formulas/chemistry/college/w6yk9tjlzf5rzpmm84cvo2ovj2r568pebq.png)
Thus, we can conclude that for the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equilibrium constant expression is K = [ H+] [NO2-] / [HNO2].