Answer:
T=846K
Step-by-step explanation:
We can use the formula for the Ideal Gas Equation, which is

P=pressure
V=volume
n=mols of gas
R=constant (0.08201 L-atm/mol-K)
T=temperature
We will have to rearrange to equation to find temperature, which is
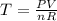
Time to solve:
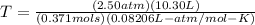
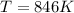
If you want to convert to Celsius, subtract 273 to your answer, which will get you 573ºC.