Answer:
glucose<NaCl<CaCl2
Step-by-step explanation:
Hello there!
In this case, since the general equation for the calculation of the freezing point depression is:
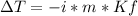
Whereas m and Kf are the same for the given salts, then we conclude that the greatest freezing point is exhibited by the salt with the greatest Van't Hoff's factor and the smallest freezing point with the smallest Van't Hoff's factor. In such a way, since this factor is equal to the number of ionized species, we infer that CaCl2 has i=3, glucose i=1 (nonionizing) and NaCl i=2; therefore, the order from smallest to greatest is:
glucose<NaCl<CaCl2
Regards!