Answer:
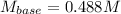
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to solve this problem by firstly writing the chemical reaction between hydrochloric acid and barium hydroxide:
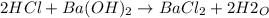
It means there is 2:1 mole ratio of the acid to base, and we are able to write the following mole ratio, then written in terms of volumes and concentrations:
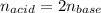
Thus, we obtain the following:
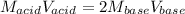
Then, we solve for the concentration of the base and consequently plug in the given data to obtain:
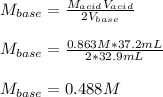
Regards!