Answer:
, The partial pressure of O2 is = 21 atm
Step-by-step explanation:
The concept is the pressure exerted by each gas is independent of one another or in other words independent of the pressure exerted by all other gases present.
This means that total pressure is the sum of the individual pressure of each gas by dalton's law.
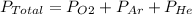
So, putting the given values we get
43 = P_O2 +15+7
P_O2 = 21 atm
Therefore, The partial pressure of O2 is = 21 atm