Answer:
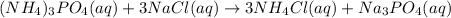
Step-by-step explanation:
Hello there!
In this case, according to the given information, we can set up the appropriate chemical equation when ammonium phosphate reacts with sodium chloride in aqueous solution:
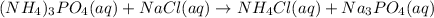
Which stands for a double replacement reaction, whereby ammonium changes phosphate to chloride and sodium changes chloride to phosphate on the products side. In addition, we can balance the aforementioned equation as shown below:
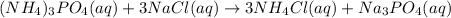
Regards!