Answer:
Solid.
Step-by-step explanation:
Hello there!
In this case, according to the given description of the freezing and boiling point of oxygen, it turns out possible for us to figure out the phase of oxygen at 35 K by just subtracting 273:
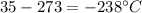
In such a way, since this temperature is lower than its freezing point of -219 °C, we infer that such sample will be solid.
Regards!