Answer: The approximate pressure of the gas after it is heated to 278 K is 0.468 atm.
Step-by-step explanation:
Given:
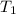 = 178 K,
= 178 K,
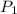 = 0.3 atm
= 0.3 atm
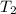 = 278 K,
= 278 K,
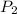 = ?
= ?
According to Gay Lussac law, at constant volume the pressure of a gas is directly proportional to the temperature.
Formula used to calculate the pressure is as follows.
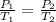
Substitute the values into above formula is as follows.
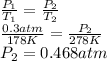
Thus, we can conclude that the approximate pressure of the gas after it is heated to 278 K is 0.468 atm.