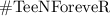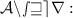
The Chemical equations has to be written in balanced form because According to the law of Conservation of mass - mass can neither be created nor be destroyed during a chemical reaction, so the mass of reactants need to be equal to the mass of products formed.
And, In order to equalize the mass on both sides we write it in balanced form.
_____________________________