Answer:
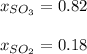
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible for us to solve this problem by using the Dalton's law which allows us to calculate the mole fractions by dividing the partial pressures over the total pressure. In such a way, we plug in the given pressures of sulfur trioxide (SO3) and sulfur dioxide (SO2) to obtain:

Best regards!