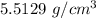Answer:
"5.5129 g/cm³" is the appropriate solution.
Explanation:
According to the question, the values are:
Iron's density,
= 7.874 g/cm³
Base edge length,
= 4 cm
Base edge width,
= 9 cm
Height,
= 5 cm
Mass,
= 425 g
As we know,
The Density of rectangular pyramid will be:
=
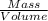
or,
=
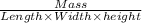
On putting all the given values, we get
=
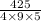
=
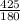
=
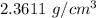
hence,
The difference will be:
=
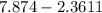
=