Answer:
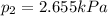
Step-by-step explanation:
Hello there!
In this case, according to the Dalton's law, which states that the total pressure is equal the sum of the pressures of the gases in the mixture, we write the following for this system:
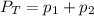
Thus, we solve for the partial pressure of the gas #2 as shown below:
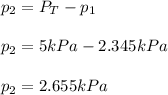
Best regards!