Answer:
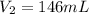
Step-by-step explanation:
Hello there!
In this case, since the equation for the calculation of dilutions is:
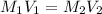
Whereas M is the molarity and V the volume, because the final concentration is lower than the initial. Thus, since we are asked to calculate the final volume, we solve for V2 as follows:
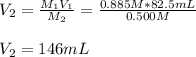
Best regards!