Answer:
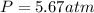
Step-by-step explanation:
Hello there!
In this case, since the Henry's law is shown below:
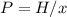
Whereas x is the mole fraction, H the Henry's constant and P the pressure. In such a way, we plug in the aforementioned first two to obtain the corresponding pressure:
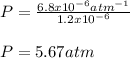
Regards!