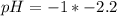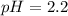Answer:
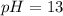 --- Oven Cleaner
--- Oven Cleaner
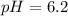 --- Water
--- Water
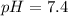 --- Blood
--- Blood
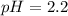 --- Vinegar
--- Vinegar
Explanation:
The given molar concentration are:[Missing from the question]
Oven Cleaner: [h+] =
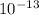
Water: [h+] =
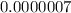
Blood: [h+] =
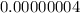
Vinegar: [h+] =
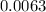
Required
Determine the pH
pH is calculated using:
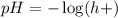
So, we have:
Oven Cleaner:
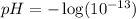
Using a calculator, we have:
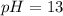
Water:
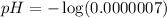
Using a calculator, we have:
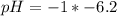
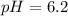
Blood:
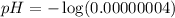
Using a calculator, we have:
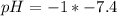
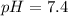
Vinegar:
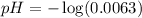
Using a calculator, we have: