Answer:
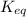 at the temperature of the experiment is 0.56.
at the temperature of the experiment is 0.56.
Step-by-step explanation:
Moles of
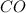 = 0.35 mole
= 0.35 mole
Moles of
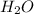 = 0.40 mole
= 0.40 mole
Volume of solution = 1.00 L
Initial concentration of
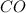 =
=
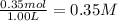
Initial concentration of
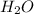 =
=
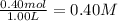
Equilibrium concentration of
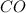 =
=
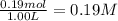
The given balanced equilibrium reaction is,
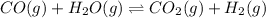
Initial conc. 0.35 M 0.40 M 0 M 0M
At eqm. conc. (0.35-x) M (0.40-x) M (x) M (x) M
Given: (0.35-x) = 0.19
x= 0.16 M
The expression for equilibrium constant for this reaction will be,
![K_(eq)=([CO_2]* [H_2])/([CO]* [H_2O])](https://img.qammunity.org/2022/formulas/chemistry/college/47rj026c7xnop9d4ukdl0307l0ouwi8zdw.png)
Now put all the given values in this expression, we get :
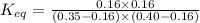
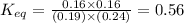
Thus
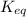 at the temperature of the experiment is 0.56.
at the temperature of the experiment is 0.56.