Given :
An ideal gas contained in a cylinder with the volume 34.4L, temperature 667K and pressure 2 atm.
To Find :
What will be pressure of the gas if it is heated at 1000k and the volume becomes 50L.
Solution :
We know, by ideal gas equation :
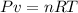
Now, in the given question only their are two state and also their is no leakage so amount of gas is also constant.
So,
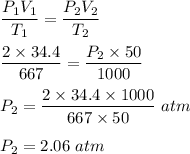
Therefore, the pressure of gas is 2.06 atm.