Answer:
Molality of the diluted sample is 0.778 M
Step-by-step explanation:
Given -
Molality of the original solution = 3.5 M
Volume of the original solution = 20 mL
Volume of the diluted solution = 90 mL
Molality of the diluted sample = X
As we know
Molality of the original solution * Volume of the original solution = Volume of the diluted solution * Molality of the diluted sample
Substituting the given values, we get -
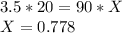 M
M
Molality of the diluted sample is 0.778 M