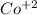Answer:
a
Step-by-step explanation:
The formation of ion occurs when an atom that is said to be neutral gains or losses electrons.
At the time it gains electrons, it is regarded that a negative ion (anion) is formed.
When it loses electron, it is regarded that a positive ion (cation) is formed.
Atomic number = No of protons and electrons occurring in a neutral atom.
Given that:
Protons = 14
electron = 18
Net Charge = no of proton - no of electron
= 14 - 18
= -4
Mass number = 14 + 15 = 29
So, the chemical symbol =
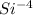
For ion with
27 proton, 32 neutrons and 25 electrons
Net charge = 27 - 25
= +2
Mass number = 27 + 32 = 59
Thus, the chemical symbol =