Answer:
Mass of CaCl2 = 4.1625 grams
Step-by-step explanation:
We will recall that:
For solution: Molarity =
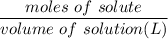
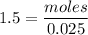
Moles = 0.0375 moles
Mass = no of moles × molar mass(MM)
Molar mass of CaCl2 = 111 g/mol
Mass = 0.0375 mol × 111 g/mol
Mass of CaCl2 = 4.1625 grams