Answer:
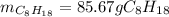
Step-by-step explanation:
Hello there!
In this case, according to the given combustion reaction of octane, it is possible for us to perform the stoichiometric method in order to calculate the mass of octane that is required to consume 300.0 g of oxygen by considering the 2:25 mole ratio, and the molar masses of 114.22 g/mol and 32.00 g/mol respectively:
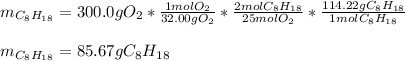
Regards!