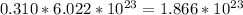Answer:
(a) 0.294 mol silver =
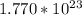
(b) 8.98 * 10-3 mol sodium chloride -
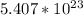
(c) 23.3 mol carbon dioxide =
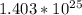
(d) 0.310 mol nitrogen (N2) =
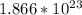
Step-by-step explanation:
In one mole there are
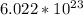 atoms/molecules
atoms/molecules
(a) 0.294 mol silver =
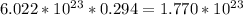
(b) 8.98 * 10-3 mol sodium chloride -
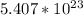
(c) 23.3 mol carbon dioxide =
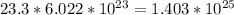
(d) 0.310 mol nitrogen (N2) =