Answer:
H₂
Step-by-step explanation:
To solve this question we must find, as first, find the molar mass of the homonuclear diatomic gas using Graham's law. With the molar mass we can identify this gas
Graham's law:
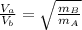
Where V is the speed of the gases and m the molar mass of those:
As Va is 3.98 times Vb (And mB is molar mass of oxygen gas = 32g/mol)
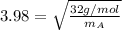
15.84 = 32g/mol / mA
mA = 2.02g/mol
As is a homonuclear diatomic gas, the molar mass of the atom is 1.01g/mol. Thus, the gas is:
H₂