Answer:
Step-by-step explanation:
✏The fizz in the club soda, the foaming effect in the washing soda are all due to carbonate ions. Carbonate is a salt of carbonic acid possessing the molecular formula CO32–. The main group of elements used as carbonates are alkali and alkaline earth metals.
Formula:
✏
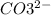
ʰᵒᵖᵉ ⁱᵗ ʰᵉˡᵖˢ