Answer:
3.67
Step-by-step explanation:
Step 1: Write the balanced equation
CO₂(g) + H₂(g) = CO(g) + H₂O(g)
Step 2: Calculate the value of the pressure equilibrium constant (Kp)
The concentration equilibrium constant (Kc) is 3.67.
We can calculate the value of the pressure equilibrium constant using the following expression.
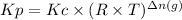
where,
R: ideal gas constant
T: absolute temperature
Δn(g): moles of gaseous products - moles of gaseous reactants
Δn(g) = 2 mol - 2 mol = 0
Then,
Kp = Kc × (R × T)⁰ = Kc = 3.67