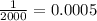Answer:
Molarity of a solution where one mole of NaOH is added to 2000mL of water is 0.0005
Step-by-step explanation:
Molarity is the equal to number of moles of solute divided by total volume of solvent
Given -
Number of moles of solute i.e NaOH = 1
Total volume of solvent i.e water = 2000 mL
Molarity