Answer:
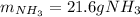
Step-by-step explanation:
Hello there!
In this case, in agreement to the given chemical reaction, it is possible for us to calculate the mass of NH3 required to remove 57.0 g NO via the stoichiometry based off the 4:6 mole ratio between them:
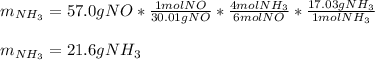
Best regards!