Answer:
a) specific work output of the actual turbine is 73.14 Btu/lbm
b) the amount of specific entropy generation during the irreversible process is 0.050416 Btu/lbm°R
c) Isentropic efficiency of the turbine is 70.76%
Step-by-step explanation:
Given the data in the question;
For an adiabatic turbine; heat loss Q = 0
For Initial State;
p₁ = 120 psia
T₁ = 500°F = 959.67°R
from table; { Gas Properties of Air }
At T₁ = 959.67°R
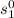 = 0.74102 Btu/lbm°R
= 0.74102 Btu/lbm°R
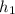 = 230.98 Btu/lbm
= 230.98 Btu/lbm
For Finial state;
p₂ = 15 psia
T₂ = 200°F = 659.67°R
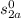 = 0.64889 Btu/lbm°R
= 0.64889 Btu/lbm°R
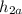 = 157.84 Btu/lbm
= 157.84 Btu/lbm
we know that R for air is 0.06855 Btu/lbm.R
a)
The specific work output of the actual turbine Wₐ is;
W
 =
=
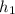 -
-
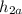
we substitute
W
 = 230.98 - 157.84
= 230.98 - 157.84
W
 = 73.14 Btu/lbm
= 73.14 Btu/lbm
Therefore, specific work output of the actual turbine is 73.14 Btu/lbm
b)
amount of specific entropy generation during the irreversible process.
To determine the entropy generation
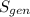 ;
;
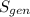 = ΔS =
= ΔS =
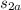 -
-
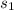 =
=
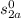 -
-
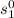 - R ln(
- R ln(
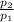 )
)
we substitute in our values
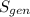 = 0.64889 - 0.74102 - 0.06855 ln(
= 0.64889 - 0.74102 - 0.06855 ln(
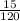 )
)
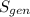 = 0.64889 - 0.74102 + 0.1425457
= 0.64889 - 0.74102 + 0.1425457
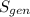 = 0.050416 Btu/lbm°R
= 0.050416 Btu/lbm°R
Therefore, the amount of specific entropy generation during the irreversible process is 0.050416 Btu/lbm°R
c)
Isentropic efficiency of turbine η
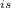
η
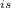 = {actual work output] / [ ideal work output ] = (
= {actual work output] / [ ideal work output ] = (
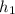 -
-
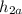 ) / (
) / (
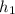 -
-
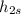 )
)
Now, for an ideal turbine;
ΔS = 0 =
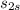 -
-
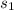
so,
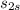 - s₁ =
- s₁ =
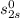 -
-
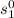 - R ln(
- R ln(
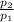 )
)
0 =
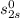 -
-
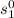 - R ln(
- R ln(
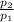 )
)
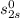 =
=
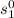 + R ln(
+ R ln(
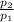 )
)
we substitute
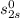 = 0.74102 + 0.06855 ln(
= 0.74102 + 0.06855 ln(
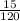 )
)
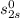 = 0.74102 - 0.1425457
= 0.74102 - 0.1425457
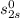 = 0.59847 Btu/lbm°R
= 0.59847 Btu/lbm°R
Now, from table; { Gas Properties of Air }
At
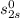 = 0.59847 Btu/lbm°R;
= 0.59847 Btu/lbm°R;
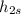 = 127.614 Btu/lbm
= 127.614 Btu/lbm
η
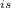 = [(
= [(
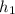 -
-
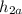 ) / (
) / (
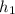 -
-
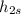 )] × 100%
)] × 100%
we substitute
η
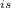 = [( 230.98 - 157.84 ) / ( 230.98 - 127.614 )] × 100%
= [( 230.98 - 157.84 ) / ( 230.98 - 127.614 )] × 100%
η
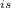 = [ 73.14 / 103.366] × 100%
= [ 73.14 / 103.366] × 100%
η
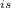 = 0.70758 × 100%
= 0.70758 × 100%
η
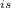 = 70.76%
= 70.76%
Therefore, Isentropic efficiency of the turbine is 70.76%