Answer:
12.7mol Na.
Step-by-step explanation:
Hello there!
In this case, according to the concept of mole, which stands for the amount of substance, we can recall the concept of Avogadro's number whereby we understand that one mole of any substance contains 6.022x10²³ particles, for the given atoms of sodium, we can calculate the moles as shown below:
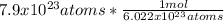
Thus, by performing the division we obtain:
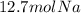
Regards!