Answer:
The molarity of the solution is 0.016
Step-by-step explanation:
Molar concentration is a measure of the concentration of a solute in a solution.
Molarity (M) or Molar Concentration is the number of moles of solute that are dissolved in a certain volume.
Molarity is determined by dividing the moles of the solute by the volume of the solution:
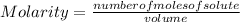
Molarity is expressed in units
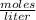 .
.
Being 169.87 g / mole the molar mass of AgNO₃, then you can apply the following rule of three: if 169.87 grams are contained in 1 mole, 6.87 grams are contained in how many moles?
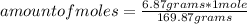
amount of moles= 0.04 moles
Then:
- number of moles of solute: 0.04 moles
- volume: 2.50 L
Replacing:
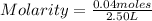
Solving:
Molarity= 0.016
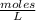
The molarity of the solution is 0.016